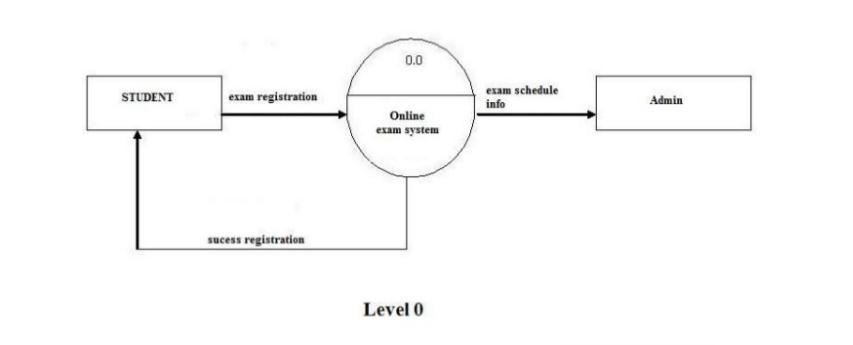
Electrolysis of solutions - Electrolysis - AQA - GCSE.
What Is the Test for Hydrogen Gas? Testing for hydrogen gas involves heating hydrogen gas over a flame and listening for a distinctive pop. Because hydrogen is highly flammable, it will react to the heat. Using a flame is the best method of testing for the presence of hydrogen. Hydrogen has no color or smell and does not react to litmus paper.The main safety consideration is making certain hydrogen gas isn't allowed to mix with oxygen in the air. Nothing bad will happen if it does, but the resulting air-hydrogen mixture is much more flammable than hydrogen on its own because it now contains oxygen, which will act as an oxidizer.WRITING IONIC EQUATIONS FOR REDOX REACTIONS.. Manganate(VII) ions, MnO 4-, oxidise hydrogen peroxide, H 2 O 2, to oxygen gas. The reaction is done with potassium manganate(VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. During the reaction, the manganate(VII) ions are reduced to manganese(II) ions.
Hydrogen reacts very quickly with oxygen to form water. Oxygen. Oxygen supports combustion so a good method of testing for oxygen is to take a glowing splint and place it in a sample of gas, if it re-ignites the gas is oxygen. This is a simple but effective test for oxygen.Acids react with most metals and, when they do, a salt is produced. But unlike the reaction between acids and bases, we do not get water. Instead we get hydrogen gas. This is the general word.

Hydrogen is a colorless, odorless, nonmetallic, tasteless, highly flammable diatomic gas with the molecular formula H2. With an atomic weight of 1. 00794, hydrogen is the lightest element. Besides the common H1 isotope, hydrogen exists as the stable isotope Deuterium and the unstable, radioactive isotope Tritium. Hydrogen is the most abundant.